Received: Wed 15, May 2024
Accepted: Mon 10, Jun 2024
Abstract
Background: Pancreatic adenosquamous cell carcinoma (PASC) is an extremely rare pathological subtype of pancreatic cancer, accounting for approximately 1-2.9% of all reported cases of pancreatic ductal adenocarcinoma. It has a worse prognosis compared to pancreatic ductal adenocarcinoma and is particularly challenging to diagnose preoperatively. In this report, we retrospectively analyzed a case of predominantly squamous cell carcinoma in PASC treated at the First Affiliated Hospital of Soochow University and reviewed relevant literature.
Case Presentation: The patient, a 71-year-old Chinese male, was admitted with a chief complaint of "left upper abdominal pain for over two months." The pain was intermittent and dull, without other discomforts such as nausea, vomiting, chest tightness, or dyspnea. Based on the patient's medical history, physical examination, laboratory results, and imaging findings, the patient was diagnosed with a pancreatic occupying lesion and hypertension. After extensive communication with the patient and their family, the patient underwent distal pancreatectomy, splenectomy, lymph node dissection, and pancreatic repair. The postoperative pathological results indicated that the adenosquamous cell carcinoma in the body and tail of the pancreas was predominantly composed of squamous cell carcinoma component. The patient received adjuvant concurrent chemoradiotherapy but developed recurrent high fever after 10 months, leading to the discontinuation of chemotherapy. Subsequent CT scan revealed extensive liver metastasis from the pancreatic adenosquamous cell carcinoma, and the patient eventually died 12 months after the surgery.
Conclusion: Adenosquamous cell carcinoma is an extremely rare pathological subtype of pancreatic malignancy, exhibiting a more aggressive behavior and poorer prognosis compared to ductal adenocarcinoma. Surgical resection is the preferred treatment option for this disease. Adjusting the chemotherapy regimen as needed and in a timely manner, such as incorporating platinum-based drugs and combining radiotherapy with PD-L1 immunotherapy, may further improve patient outcomes.
Keywords
Pancreatic adenosquamous cell carcinoma, squamous cell carcinoma, diagnosis, treatment
1. Background
It has been reported that over 90% of pancreatic cancers are ductal adenocarcinomas, while adenosquamous carcinoma is an extremely rare pathological type among malignant pancreatic tumors. Adenosquamous carcinoma exhibits a higher degree of malignancy compared to ductal adenocarcinoma and is associated with a poorer prognosis. Scholars believe that adenosquamous carcinoma is more commonly found in areas such as the anus, intestines, esophagus, uterus, cervix, and vagina, and is rare in the pancreas [1]. The histological composition of this pathological type mainly consists of a mixture of adenocarcinoma and squamous cell carcinoma (SCC), with Komatsu H and others suggesting that the proportion of squamous epithelium in PASC tissue should be ≥30% [2], while Alwaheeb S and others suggest that the presence of squamous epithelium in tumor tissue is sufficient to diagnose pancreatic adenosquamous carcinoma [3]. Hence, the origin of squamous cell carcinoma has become a topic of common concern among scholars. Cylwik B and others believe that squamous metaplasia of ductal cells can occur in patients with chronic pancreatic inflammation, after stent placement in the main pancreatic duct, and in patients with benign pancreatic cyst walls [4-6]. Here, we report a case of predominantly squamous cell carcinoma in pancreatic adenosquamous cell carcinoma and provide a literature review.
2. Case Presentation
The patient was a 71-year-old Chinese male who was admitted to the hospital for "left upper abdominal pain for over two months." The pain was intermittent and dull, without other discomfort such as nausea, vomiting, chest tightness, or dyspnea. Subsequently, the patient sought medical attention at the Mudu Town People's Hospital and underwent a CT scan, which indicated a mass lesion in the pancreas. The patient was then referred to our hospital for surgical treatment. Upon admission, the outpatient diagnosis was "pancreatic mass lesion." Physical examination revealed weakly positive tenderness in the left upper abdomen. The patient's appetite, sleep, bowel movements, and urination had been normal since the onset of the illness, but there was a weight loss of approximately 4 kg in the past 20 days. The patient had a history of hypertension for over eight years, for which he regularly took antihypertensive medications, and his blood pressure was well controlled. Initial laboratory tests for the patient are shown in (Table 1).
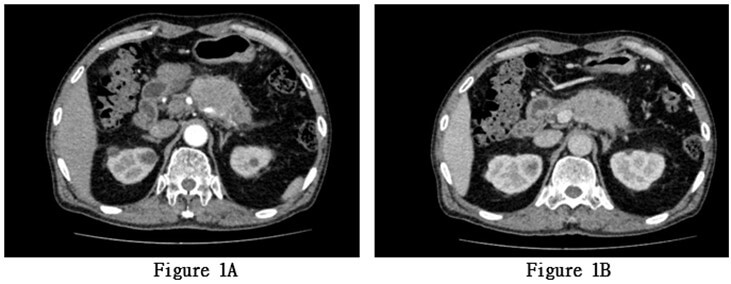
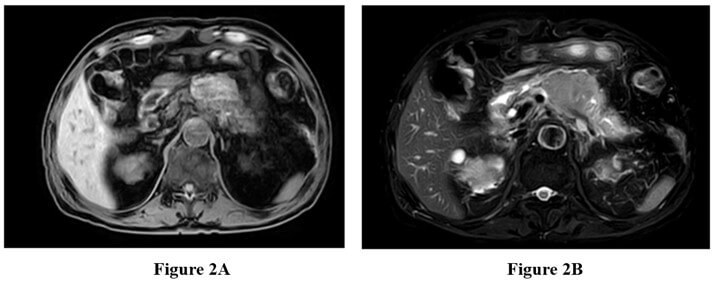
The patient underwent a complete abdominal enhanced CT scan after admission. The results showed a soft tissue mass in the body of the pancreas, measuring approximately 58 × 44 mm. Pancreatic malignancy was suspected, with the tumor encasing the splenic artery and vein. There was dilation of the pancreatic tail duct with minimal fluid accumulation in the pancreatic and pelvic regions. Multiple enlarged lymph nodes were observed around the mass, pancreaticoduodenal region, hepatic hilum, and retroperitoneum (Figures 1A & 1B). Subsequently, the patient underwent a complete abdominal enhanced MRI+MRCP. The results revealed a mass in the pancreatic body and tail with slightly high T1 signal, slightly low T2 signal, significant high signal on DWI, low ADC signal, and less enhancement compared to the surrounding normal pancreas, indicating pancreatic malignancy. Multiple enlarged lymph nodes were also observed around the pancreas and retroperitoneum (Figures 2A & 2B).
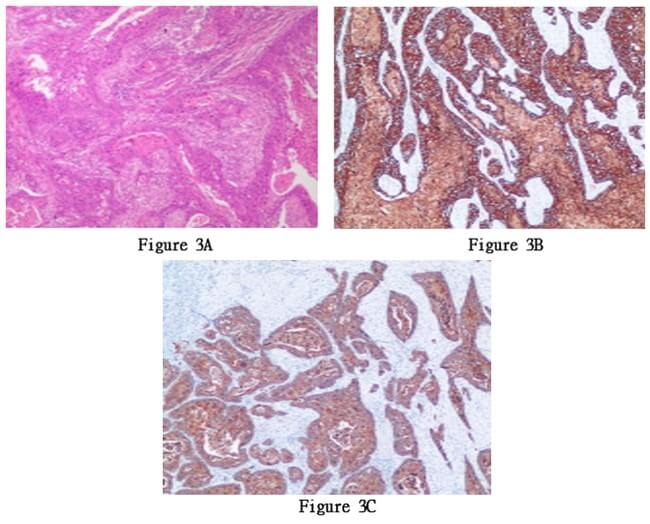
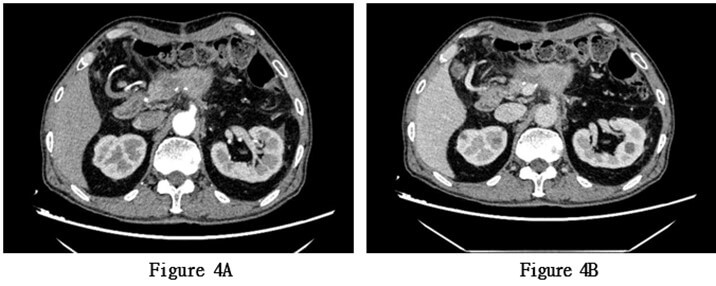
Based on the patient's medical history, physical examination, laboratory test results, and imaging findings, the patient was diagnosed with a pancreatic space-occupying lesion and hypertension. After extensive communication with the patient's family, they decided to proceed with pancreatic body and tail resection, splenectomy, abdominal lymph node dissection, and pancreatic repair on May 26, 2022, under general anesthesia. The surgery lasted for 3 hours and 45 minutes. The intraoperative findings revealed a tumor measuring approximately 6 × 4 cm, closely adhering to the splenic artery. The splenic artery was carefully separated from the tumor, and the intraoperative blood loss was approximately 600 ml. The surgery proceeded smoothly. Postoperatively, an increased amylase level in the drainage fluid suggested pancreatic fistula, which was managed through active drainage and close monitoring of fluid volume and color. The condition improved over time, and subsequent regular examinations of amylase levels in the drainage fluid were normal. The patient's general condition was good after surgery, and they were discharged on the sixth day postoperatively. The pathological examination of the surgical specimen revealed adenosquamous (with squamous cell carcinoma as the dominant component) in the pancreatic body and tail, invading the surrounding adipose tissue. No tumor involvement was observed at the surgical margins, and no lymph node metastasis was identified in the peripancreatic, splenic, or posterior abdominal lymph nodes (0/1, 0/13, 0/1). Immunohistochemistry results were positive for MLH1, PMS2, MSH6, MSH2, CK19, CK5/6, and EGFR, and negative for Her2. The Ki-67 index was 90% (Figures 3A-3C). Three months postoperatively, an implantable venous access port was placed in the patient for intravenous access. Chemotherapy with "albumin-bound paclitaxel 180mg + gemcitabine 1400mg" was initiated on August 2, 2022, after excluding contraindications for chemotherapy. The regimen was administered once a week, with a two-week interval between every three cycles. A follow- up abdominal enhanced CT scan three months after surgery showed partial absorption of the local infiltration and effusion in the surgical area and abdominal cavity compared to previous scans. There was a slightly increased density in the soft tissue at the pancreatic body surgical site, possibly indicating hematoma organization (Figures 4A & 4B).
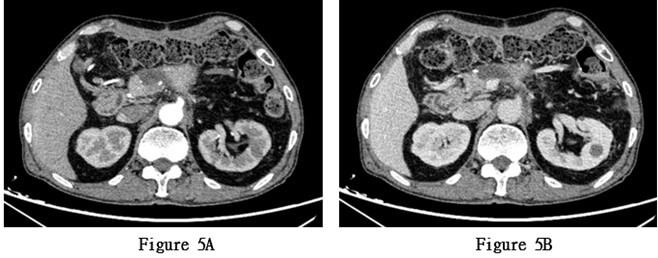
During the third and ninth cycles of chemotherapy, the patient exhibited significant leukopenia and neutropenia, likely due to chemotherapy-induced bone marrow suppression. After receiving treatment to increase white blood cell count, the blood routine examination returned to normal. The patient continued to receive chemotherapy with albumin-bound paclitaxel 180 mg + gemcitabine 1400 mg, along with supportive therapies for antiemesis, gastric protection, and liver protection. During the fifth cycle of hospitalization for chemotherapy, a follow-up abdominal enhanced CT scan revealed a slightly increased density in the soft tissue at the pancreatic body surgical site and newly developed cystic lesions, suggesting the possibility of cyst formation (Figures 5A & 5B). Subsequently, the patient's general condition deteriorated, with increased abdominal pain and fatigue. After a comprehensive evaluation by a multidisciplinary team, considering the postoperative pathological results and relevant auxiliary examinations, tumor recurrence was highly suspected. The patient was recommended to undergo combined chemotherapy and radiotherapy.
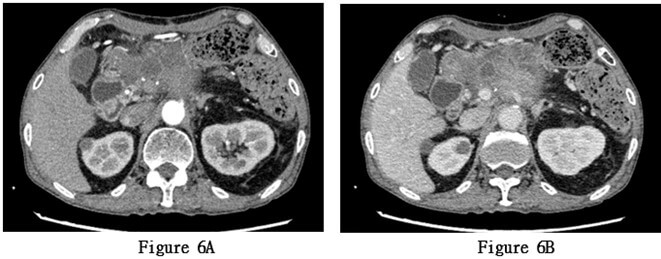
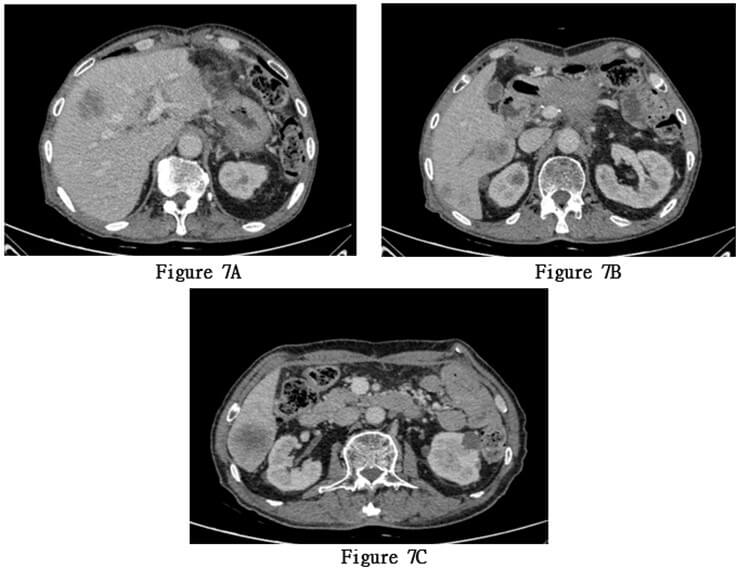
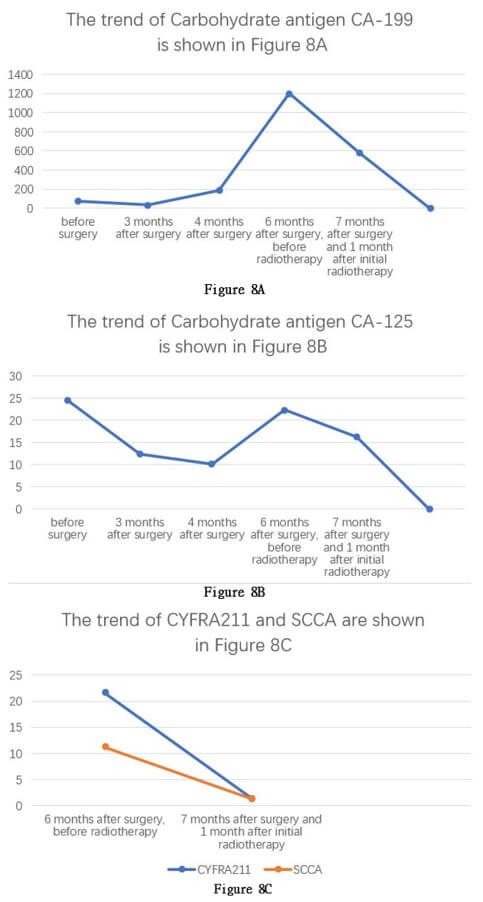
Starting from November 18, 2022, the patient received concurrent chemoradiotherapy, and the physical performance status was assessed as grade 1 upon admission. The initially planned local radiotherapy regimen for the patient is 25 sessions of radiation therapy targeting the primary lesion area, with a dose of 50Gy per session, concurrently accompanied by adjuvant chemotherapy with tegafur. A month after initiating combined chemotherapy and radiotherapy, serum tumor markers had decreased compared to previous levels, and the patient's physical condition improved. However, the patient experienced recurrent high fever, with temperatures ranging from 38.5 to 40 degree Celsius, ten months post-surgery. Anti- tumor treatment was forced to be discontinued and a follow-up CT scan showed extensive metastasis of pancreatic squamous cell carcinoma to the liver (Figures 7A-7C). The patient died 12 months after the surgery.
3. Discussion
There are currently three most widely recognized theories on the origin of squamous cell carcinoma. The first theory is that chronic pancreatic inflammation or tumor obstruction of the pancreatic duct can lead to squamous metaplasia of pancreatic duct cells, eventually leading to PASC [7, 8]. The second theory, widely circulated, is the collision theory, which proposes that two histologically distinct and functionally independent tumors in the pancreas coexist and merge, leading to the occurrence of adenosquamous carcinoma [8-10]. However, paramythiotis D and others found the same KRAS mutation in both types of tumors, suggesting a possible common origin, thus casting doubt on the collision hypothesis [11]. The third theory suggests the presence of multipotent stem cells in the tissue, with some differentiating into adenocarcinoma and others into SCC, resulting in tumors containing both cell types [8, 12].
Generally speaking, the clinical manifestations of pancreatic adenosquamous carcinoma (PASC) lack specificity compared to pancreatic ductal adenocarcinoma. Patients often present with worsening jaundice or upper abdominal pain. In this case, the patient was admitted due to "left upper abdominal pain for over two months." Preoperative examinations all misdiagnosed the condition as ductal adenocarcinoma, but postoperative pathology reported adenosquamous carcinoma predominantly composed of squamous cell carcinoma in the body and tail of the pancreas. Currently, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and postoperative pathological examination remain the gold standard for diagnosing PASC and PSCC, but EUS-FNA may have the high risk of unsuccessful biopsy and absence of typical squamous metaplasia in the sampled tissue, greatly increasing the difficulty of diagnosis.
Boyd CA et al. found that the median overall survival after surgery for pancreatic adenosquamous carcinoma patients is 12 months, while it is 16 months for pancreatic ductal adenocarcinoma patients, with a statistically significant difference [13]. The median overall survival of patients with pancreatic squamous cell carcinoma postoperatively is lower, with the patient in this case having a postoperative survival time of exactly 12 months. Some studies have reported that the progression of squamous cell carcinoma is characterized by vascular invasion rather than lymph node metastasis. Compared to pancreatic ductal adenocarcinoma, PASC is more prone to invading surrounding blood vessels and metastasizing to organs such as the liver and lungs [14]. In this case, intraoperatively, the tumor was found to be closely adherent to the hepatic artery, and postoperative pathology indicated negative lymph nodes around the tumor, and the patient ultimately succumbed to the malignant tumor with extensive liver metastasis confirming these findings. It also suggests that the higher the proportion of squamous epithelial carcinoma components in pancreatic cancer, the worse the patient's prognosis.
Currently, there is no definitive standard for treating pancreatic adenosquamous carcinoma. Some studies suggest that both the radiotherapy group and the chemotherapy group have better prognosis than the group that receives neither radiotherapy nor chemotherapy. Additionally, combined chemotherapy and radiotherapy show better survival outcomes than receiving a single treatment modality. In the same study, it was found that although the survival rate of the group receiving combined chemotherapy and radiotherapy was higher than the single treatment group, there was no statistically significant difference in survival rates between the chemotherapy and radiotherapy subgroups [15]. Therefore, we conclude that surgical treatment followed by adjuvant chemotherapy and radiotherapy remains the preferred treatment approach.
Regarding the selection of chemotherapy drugs for PASC, platinum-based drugs have been shown to improve outcomes in some squamous cell carcinomas, such as esophageal and ovarian cancers [16]. A recent study showed that the median survival time of patients with pancreatic adenosquamous carcinoma who received platinum-based drugs for adjuvant therapy was significantly higher than that of patients who received conventional gemcitabine combined with albumin paclitaxel therapy [16]. Therefore, after we learned of the patient's postoperative pathology suggestive of PASC, we can improve the patient's chemotherapy regimen by adding platinum-based drugs to improve prognosis. Postoperative adjuvant radiotherapy as a treatment option for PASC can improve postoperative survival time. Sun et al. believed that there was no statistically significant difference in postoperative treatment outcomes between patients with pancreatic ductal adenocarcinoma who received postoperative combined chemotherapy and radiotherapy and patients who received only postoperative chemotherapy. However, the median postoperative survival time of PASC patients who received combined chemotherapy and radiotherapy can reach 31 months, which is significantly better than that of patients who only received postoperative chemotherapy, indicating that radiotherapy is more sensitive to SCC in PASC [17].
So far, research on targeted therapy for PASC is still limited. It has been proven that EGFR is overexpressed in the vast majority of pancreatic cancers and has an important impact on the metastasis and recurrence of pancreatic cancer. Erlotinib, an EGFR inhibitor, has been shown to have a statistically significant impact on the median survival time of patients [18], but the combination of gemcitabine and erlotinib only improves the average survival time of patients by two weeks compared to the use of gemcitabine alone [19]. Nowadays, immunotherapy has shown significant effects in cancers such as lung cancer and melanoma, but treatment of CTLA4 and PD-1/PD-L1 immune checkpoints in pancreatic cancer and other immunotherapeutic methods have not been successful [20]. However, PD-L1's immune checkpoint expression in PASC suggests that PD-L1 may be effective in treating PASC patients [21]. The patient in this case also received immunotherapy targeting PD-L1.
4. Conclusion
Pancreatic adenosquamous carcinoma is rarer than pancreatic ductal adenocarcinoma, making its diagnosis challenging and prognosis worse. Early detection, diagnosis, and treatment are crucial for patients with this type of disease, especially R0 surgical resection. Simultaneously, postoperatively, the chemotherapy regimen was adjusted as needed and in a timely manner, such as adding platinum-based drugs, combined with radiotherapy and PD-L1 immunotherapy, can further improve patient prognosis [22]. Due to the rarity of pancreatic adenosquamous carcinoma, there is a lack of reports on this type of disease, and further large-scale clinical and basic research on PASC is needed to gain a more comprehensive understanding.
Acknowledgements
Not applicable.
Author Contributions
Nuwa Wu conceived the idea and wrote the manuscript. Jiayue Zou and Wentao Huang collected patient data and materials. Lei Qin and Nuwa Wu followed up with patients and performed surgeries. All authors participated in this research, designing and coordinating it, and all authors contributed to this research.
Funding
None.
Availability of Data and Materials
Not applicable.
Ethics Approval and Consent to Participate
All procedures used in this research were approved by the Ethical Committee of the First Affiliated Hospital of Soochow University.
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Competing Interests
None.
Abbreviations
PASC: Pancreatic Adenosquamous Cell Carcinoma CT: Computed Tomography PD-L1: Programmed Cell Death 1-ligand 1 SCC: Squamous Cell Carcinoma Kg: Kilogram MRI: Magnetic Resonance Imaging MRCP: Magnetic Resonance Cholangiopancreatography DWI: Diffusion Weighted Imaging ADC: Apparent Diffusion Coefficient EGFR: Epidermal Growth Factor Receptor Mg: Milligram Her2: Human Epidermal Growth Factor Receptor 2 Gy: Gray KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog EUS-FNA: Endoscopic Ultrasound-Guided Fine-Needle Aspiration CTLA4: Cytotoxic T Lymphocyte-Associated Antigen-4
REFERENCES
1. Evangelia Skafida, Xanthippi Grammatoglou, Chryssoula Glava, et al. “Adenosquamous
carcinoma of the pancreas: a case report.” Cases J, vol. 3, pp. 41,
2010. View at: Publisher Site | PubMed
2. Hirotake Komats, Shinichi Egawa, Fuyuhiko Motoi, et al.
“Clinicopathological features and surgical outcomes of adenosquamous carcinoma
of the pancreas: a retrospective analysis of patients with resectable stage
tumors.” Surg Today, vol. 45, no. 3, pp. 297-304, 2015. View at: Publisher Site | PubMed
3. S Alwaheeb, R Chetty “Adenosquamous carcinoma of the pancreas with an
acantholytic pattern together with osteoclast-like and pleomorphic giant
cells.” J Clin Pathol, vol. 58, no. 9, pp. 987-990, 2005. View at: Publisher Site | PubMed
4. B Cylwik, H F Nowak, Z Puchalski, et al. “Epithelial anomalies in
chronic pancreatitis as a risk factor of pancreatic cancer.” Hepatogastroenterology,
vol. 45, no. 20, pp. 528-532, 1998. View at: PubMed
5. L J Layfield 1, H Cramer, J Madden, et al. “Atypical squamous epithelium
in cytologic specimens from the pancreas: cytological differential diagnosis
and clinical implications.” Diagn Cytopathol, vol. 25, no. 1, pp. 38-42,
2001. View at: Publisher Site | PubMed
6. T Kubo, T Takeshita, T Shimono, et al. “Squamous-lined cyst of the
pancreas: Radiological-pathological correlation.” Clin Radiol, vol. 69,
no. 8, pp. 880-886, 2014. View at: Publisher Site | PubMed
7. D E Kardon, L D Thompson, R M Przygodzki, et al. “Adenosquamous
carcinoma of the pancreas: a clinicopathologic series of 25 cases.” Mod
Pathol, vol. 14, no. 5, pp. 443-451, 2001. View at: Publisher Site | PubMed
8. Guru Trikudanathan, Constantin A Dasanu “Adenosquamous carcinoma of the
pancreas: a distinct clinicopathologic entity.” South Med J, vol. 103,
no. 9, pp. 903-910, 2010. View at: Publisher Site | PubMed
9. J A Madura, B T Jarman, M G Doherty, et al. “Adenosquamous carcinoma of
the pancreas.” Arch Surg, vol. 134, no. 6, pp. 599-603, 1999. View at: Publisher Site | PubMed
10. J Kovi “Adenosquamous carcinoma of the pancreas: a light and electron
microscopic study.” Ultrastruct Pathol, vol. 3, no. 1, pp. 17-23, 1982.
View at: Publisher Site | PubMed
11. Daniel Paramythiotis, Filippos Kyriakidis, Eleni Karlafti, et al.
“Adenosquamous carcinoma of the pancreas: two case reports and review of the
literature.” J Med Case Rep, vol. 16, no. 1, pp. 395, 2022. View at: Publisher Site | PubMed
12. Christine G Simone, Tania Zuluaga Toro, Ellie Chan, et al.
“Characteristics and outcomes of adenosquamous carcinoma of the pancreas.” Gastrointest
Cancer Res, vol. 6, no. 3, pp. 75-79, 2013. View at: PubMed
13. Casey A Boyd, Jaime Benarroch-Gampel, Kristin M Sheffield, et al. “415
patients with adenosquamous carcinoma of the pancreas: a population-based
analysis of prognosis and survival.” J Surg Res, vol. 174, no. 1, pp.
12-19, 2012. View at: Publisher Site | PubMed
14. Sojun Hoshimoto, Nobuo Hoshi, Shoichi Hishinuma, et al. “Clinical
implications of the proliferative ability of the squamous component regarding
tumor progression of adenosquamous carcinoma of the pancreas: A preliminary
report.” Pancreatology, vol. 17, no. 5, pp. 788-794, 2017. View at: Publisher Site | PubMed
15. Yuan Fang, Ning Pu, Lei Zhang, et al. “Chemoradiotherapy is associated
with improved survival for resected pancreatic adenosquamous carcinoma: a
retrospective cohort study from the SEER database.” Ann Transl Med, vol.
7, no. 20, pp. 522, 2019. View at: Publisher Site | PubMed
16. Aaron T Wild, Avani S Dholakia, Katherine Y Fan, et al. “Efficacy of
platinum chemotherapy agents in the adjuvant setting for adenosquamous
carcinoma of the pancreas.” J Gastrointest Oncol, vol. 6, no. 2, pp.
115-125, 2015. View at: Publisher Site | PubMed
17. Sun-Yuan Lv, Min-Jie Lin, Zhao-Qun Yang, et al. “Survival Analysis and
Prediction Model of ASCP Based on SEER Database.” Front Oncol, vol. 12,
pp. 909257, 2022. View at: Publisher Site | PubMed
18. Maitham A Moslim, Max D Lefton, Eric A Ross, et al. “Clinical and
Histological Basis of Adenosquamous Carcinoma of the Pancreas: A 30-year
Experience.” J Surg Res, vol. 259, pp. 350-356, 2021. View at: Publisher Site | PubMed
19. Malcolm J Moore, David Goldstein, John Hamm, et al. “Erlotinib plus
gemcitabine compared with gemcitabine alone in patients with advanced
pancreatic cancer: a phase III trial of the National Cancer Institute of Canada
Clinical Trials Group.” J Clin Oncol, vol. 25, no. 15, pp. 1960-1966,
2007. View at: Publisher Site | PubMed
20. John P Neoptolemos, Jörg Kleeff, Patrick Michl, et al. “Therapeutic
developments in pancreatic cancer: current and future perspectives.” Nat Rev
Gastroenterol Hepatol, vol. 15, no. 6, pp. 333-348, 2018. View at: Publisher Site | PubMed
21. Masahiko Tanigawa, Yoshiki Naito, Jun Akiba, et al. “PD-L1 expression in pancreatic adenosquamous carcinoma: PD-L1 expression is limited to the squamous component.” Pathol Res Pract, vol. 214, no. 12, pp. 2069-2074, 2018. View at: Publisher Site | PubMed
22. Wen Zhang 1, Jing Zhang 2, Xijun Liang, et al. “Research advances and treatment perspectives of pancreatic adenosquamous carcinoma.” Cell Oncol (Dordr), vol. 46, no. 1, pp. 1-15, 2023. View at: Publisher Site | PubMed
