Received: Thu 08, Feb 2024
Accepted: Tue 27, Feb 2024
Abstract
Background: It is well known that edema can persist after meningioma resection, and sometimes it is not resolved after this time. This study aimed to establish the relationship between a series of variables associated with meningioma or surgery, and the duration of postoperative edema.
Methods: We conducted a retrospective study of 77 meningiomas resected at our institution between January 2016 and January 2018 with a maximum follow-up period of up to three years. The independent variables collected were demographics, tumor location, relationship with the sinuses (invasion/contact), relationship with arterial structures, deviation from the midline, volume (cm3), degree of initial edema, WHO histological classification, degree of atypia, degree of resection, previous embolization, and development of complications. The edema levels were classified according to the classification described by Ide et al. (1995): GR0, GR1, and GR2. Measurements were performed using FLAIR magnetic resonance sequences. Statistical analyses were performed using the SPSS 21.
Results: Age (p=0.003), deviation from the midline (p=0.001), and tumor volume (p<0.001) were correlated with outcome using Spearman's test. Univariate analysis revealed that the localization (p=0.016), initial edema (p<0.001), degree of atypia (p=0.019), and presence of previous embolization (p=0.037) were statistically significant. In multivariate analysis, only age, initial edema, and embolization were significant independent predictors.
Conclusion: These results suggest that the degree of initial edema, midline deviation, tumor volume, tumor location, degree of atypia, and previous embolization may be important predictors of postoperative edema duration.
Keywords
Meningioma, microsurgery, peritumoral edema, postoperative edema, size tumoral
1. Introduction
Peritumoral edema (PTE), present in intracranial neoplasms, is a critical complication that makes its management hard. The severity and quantity of PTE might also restriction surgical exposure and increase the problem of the surgical procedure [1]. Severe peritumoral edema develops in malignant brain tumors including glioblastoma or brain metastases. Tumor vessels frequently lack tight junctions, which might be usually present in everyday brain micro vessels, resulting in vasogenic edema, which could interfere with the feature of adjacent brain tissue. The edematous tissue may additionally incorporate infiltrating tumor cells [2].
Meningiomas are unique edema-generating neoplasms of the central nervous system (CNS) neoplasms. They represent about 15% of all intracranial neoplasms, are normally more-axial, and show off a benign behavior. No matter these characteristics, they can be difficult to manage due to postoperative complications. Up to 2- thirds (40-60%) of meningiomas are related to PTE, which is regularly of excessive intensity, with no obvious relationship to length or pathological functions [3]. Some authors have suggested that the presence of PTE may be related to a more difficult management however the prognostic factors that have an impact on in morbidity and mortality after surgical resection stays controversial [4].
Numerous factors had been proposed to provide an explanation for the occurrence of PTE related to meningiomas, together with tumor length, location, histological differentiation, vascularization, venous stasis, hormone receptors, secretory hobby, macrophage infiltration, micro cortical invasion, and growth factors [4]. We retrospectively analyzed a series of patients who underwent surgical resection in our department from 2016 to 2018, with the aim of elucidating the potential relationship among the characteristics of meningiomas and neurosurgical strategies that might influence the endurance of PTE.
2. Material and Methods
We retrospectively analyzed 77 histologically confirmed meningiomas that underwent microsurgical resection at our institution between January 2016 and January 2018, with a maximum follow-up period of up to three years. The demographic variables of the patients were collected, and the preoperative magnetic resonances were evaluated to examine the tumor location, the relationship with important arterial structures of the tumor, its contact or invasion with the sinuses, the deviation from the midline that it produced, the volume in cm3, the degree of edema, and previous embolization of feeders.
Postoperatively, the histological classification of the tumor was performed according to the World Health Organization (WHO) classification, degree of atypia, degree of resection, and development of complications. All patients underwent MRI within 30 days prior to surgery. The tumor volumes were obtained, approximately, using the spheroid formula (V = 4/3π × abc), where a and b correspond to the maximum perpendicular diameters obtained at the axial level and c corresponds to the height obtained by the sum of axial images where the tumor is actually present, multiplied by the thickness of the slices [5].
The tumors were classified as small (less than 15 cm3), medium (15-40 cm3), or large (greater than 40 cm3). Edema was identified as hyperintense on T2-weighted MRI or hypointense on T1-weighted MRI. PTE was classified according to the scale proposed by Ide et al. [6] based in FLAIR sequences of the MRI. The three groups were GR0, GR1, and GR2. GRO was defined as the presence of a small halo around the tumor, GR1 represented edema spreading variably by white matter tracts without involving an entire hemisphere, and GR2 represented holohemispheric or near- holohemispheric edema. The duration of postoperative edema was measured by months until its disappearance, and was used as the dependent variable.
Statistical analyses were performed using the SPSS version 11. The correlation between tumor volume and PTBE volume was examined using Pearson's correlation test. Correlations between the edema index and tumor volume, sex, existence of the peritumoral arachnoid plane, tumor interface shape, SI on T2WI, histological classification, and Ki-67 antigen index were examined using the χ2 test for univariate analysis and logistic regression for multivariate analysis. Spearman's test was used to analyze the correlation between the quantitative variables age, midline deviation, and tumor volume, and the outcome variable duration of postoperative edema were analyzed. Statistical significance was set at P <0.05.
3. Results
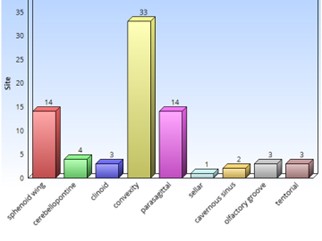
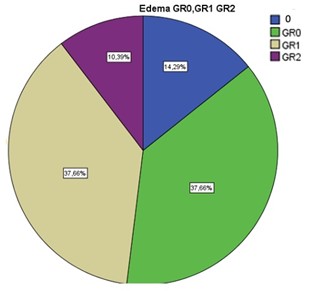
Of the 77 resected meningiomas, 53 (69%) were female and 24 (31%) were male, with a mean age of 60 years (range 23-89; median: 66 years). The most frequent tumor location was the convexity (33 patients), and within this, the frontal convexity (16 patients) represented 43% of the total. Second, the sphenoid wing and parasagittal locations were found in 16 patients each (Figure 1). The most frequent degree of preoperative edema was GR0 and GR1 with 29 patients in each group representing 38% each, 11 patients had no edema and 8 had GR2 (Figure 2).
The mean tumor volume was 21.16 cm3 (range 0.01-138.02 cm3). 57% were small tumors (less than 15 cm3), 27% were medium tumors (-15-40 cm3), and 16% were large (> 40 cm3). The majority of tumors classified as WHO grade I histology were meningiomas (32 patients, 41.6%), followed by fibrous meningiomas (15 patients, 19.5%), and meningothelial meningiomas (14 patients, 18.2%). Eleven patients were classified as WHO grade II, and there were no WHO grade III meningiomas.
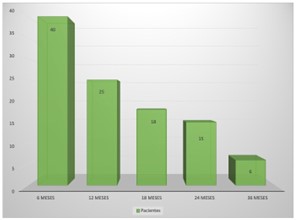
Regarding the duration of postoperative edema, at 6 months, 40 patients (51.9%) continued to have some type of edema; at 12 months, 25 patients (32.5%); at 18 months, 18 patients (23.4%); at 24 months, 15 patients (19.5%); and at 36 months, six patients (7.8%). In the 3-year follow-up period, there was no recurrence or appearance of new meningiomas (Figure 3).
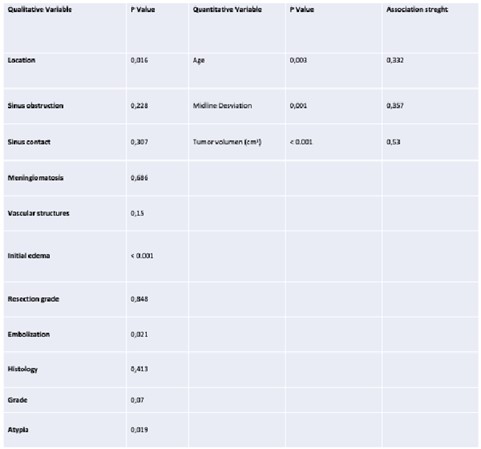
Age reached statistical significance (P =0.003), with a moderate strength of association of 0.332. The mean line deviation also reached statistical significance (0.001) with a moderate strength of association of 0.357. Tumor volume cm3 was significant (p =0.000), with a strength of association of 0.53. The remaining variables were significant for location (p=0.016), initial edema (p<0.001), degree of atypia (p=0.019), and previous embolization (p=0.021) (Table 1).
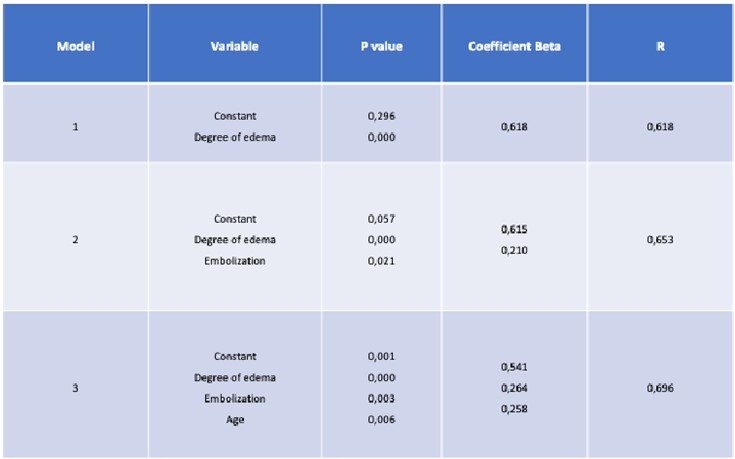
In the multivariate analysis, only age, initial edema, and embolization were statistically significant independent predictors of postoperative edema duration, with a significantly higher beta coefficient for initial edema (Table 2).
4. Discussion
Cerebral edema accompanying intracranial expansive processes is often found. This edema, which is normally vasogenic in nature, is placed in the white matter and appears much like a finger at the images. This can be a consequence of the alteration of the blood-brain barrier, which permits the passage of plasmatic macromolecules to the perivascular and extracellular area of the tumor and to the encompassing brain tissue itself, resulting in an alteration of osmotic balance and fluid retention. In gliomas, the volume of perilesional edema tends to correlate with the degree of malignancy of the procedure; the same is not true in meningiomas, in which tumors with benign histology are on occasion surrounded by intense cerebral edema [7].
Meningiomas are separated from the white matter by the arachnoid membrane, subarachnoid space, pia mater, and cerebral cortex, which constitute a dense community of neurons and glial cells proof against fluid passage. however, 60% of meningiomas are associated with PTBE [8]. It's also surprising to see how, now and again, small meningiomas, whose removal does now not generally pose technical troubles, give rise to severe cerebral edema which could seriously complicate both the surgical treatment and postoperative evolution of the patient.
In contrast to intra-axial tumors, the pathophysiology of that is attributed to disruption of the blood-brain barrier, the precise mechanism of peritumoral edema remains unknown [9]. Distinctive theories were proposed to give an explanation for its formation, including mechanical factors, consisting of tumor compression of the parenchyma that reasons cerebral ischemia [10] or compression of large veins and/or sinus veins that produce venous stasis [11]. Secretory- excretory phenomena have additionally been hypothesized with the production of edematogenic substances [12], inclusive of VEGF production [13], in addition to hydrodynamic factors that damage the blood-brain barrier [14]. More recently, Tanaka et al. [15] proposed that PTBE is probably related to hypoplasia of the tumor-draining vein. Inamura et al. [16] correlated the form of irrigation of the pial artery with peritumoral edema formation. Bitzer et al. [9] confirmed this finding and observed greater tumor invasion of the adjacent parenchyma in cases of pial irrigation.
Peritumoral edema is associated with a higher incidence of postoperative complications. patients with a preoperative edema index greater than 2 (edema index) have almost 73% chance of developing it, in comparison to 33% for patients with an index less than 1. It's also important for prognosis and recurrence [17]. Mantle [18] found a correlation between peritumoral edema and the rate of tumor recurrence. In addition, they propose a recurrence chance equation for meningiomas, where the chance of tumor recurrence = (edema cm)3 × 0.7.
In comparison, a reduced response to dexamethasone has been determined in peritumoral edema of meningiomas [19]. This reality is paradoxical if one considers that those tumors have excessive levels of glucocorticoid receptors and that the edema response to dexamethasone is directly proportional to the ranges of those receptors [12]. The effect of steroids is gradual, with an average of 50% reduction in PTBE after 7 days of treatment [20]. However, PTBE is rarely affected in association with meningiomas [21], and the hypodensity seen on CT within 1 year in 30% of patients suggests that various mechanisms by which PTBE occurs [22].
Other authors, tumor size [23], tumor location, histological type, vascularization [24], presence or absence of fenestrations in blood vessels, vascularization of tumor from the intracerebral artery determine rapid development of ability. The presence of high cell proliferation, the presence of progesterone receptors in the tumor and recently the content of tumor prostaglandins affects the edema period.
Tumor Size
Meningioma size is a determinant of the development of cerebral edema and has been described in many previous studies [11]. Go et al. [25] found a correlation between tumor size and surrounding edema, Stevens et al. [14] correlated this with tumor area. Similar to our study, these findings are important in the detection of anomalies and are associated with sizes greater than 2 cm. The similarity between the size of the tumor and the degree of edema may be due to the effective combination of the tumor in the brain. However, experimental studies have shown that the response in the form of edema is inconsistent when epidural compression is applied to the brain [26]. Edema responds well to corticosteroids, whereas meningioma edema does not. In contrast, patients with extracerebral hematomas, whether acute or chronic, rarely have cerebral edema of similar characteristics. Finally, it is true that small meningiomas can produce benign perilesional edema.
For the same reason, large meningiomas can cause compression of many cortical vessels, causing venous stasis and cerebral edema. However, the appearance of edema in meningiomas does not show any abnormalities typical of venous occlusion [25], such as focal subarachnoid hemorrhage, intracerebral hemorrhage, or similar cortical strength. Tumor size should be significant in relation to edema and duration; However, other factors must also be taken into account.
Tumor Location
There are no consistent results regarding the location associated with edema. Most of the following areas are most likely to be affected by tumor edema: convexity [27], parasagittal [28], falx [11], sphenoid wing [16], frontobasal [3], and middle fossa [3]. Less edema has also been reported in tumors occurring in the posterior fossa [15] and tentorium, which may be due to earlier onset of symptoms or lower white matter density in the posterior fossa. Other specific areas are associated with specific explanations, such as sphenoidal meningiomas of the lesser wing [12] and occlusion of the sphenoparietal sinus and tributary branches. It is worth mentioning the differences in the scales used in the literature that often prevent a certain association from being defined.
Other Features
Other explanations have associated tumor-induced damage to the cerebral cortex with the existence of edema. On the one hand, histological studies and the fact that meningiomas capture amount of contrast make us believe that there is a lack of a barrier in the meningiomatous vessels [10]. Edema can develop in the tumor itself, cross the cerebral cortex after break it and accumulate in the white matter.
Cortical distension due to large meningiomas may cause edema creating a hydraulic continuity between tumor stroma and white matter. This hypothesis is supported by the observation that brain regions with less cortical thickness in contact with meningiomas are more edematous [10]. Different histological groups have also been associated with the development of more severe edema, specifically meningothelial and transitional types. In our study, however, no correlation was found between these two parameters. However, there is a positive correlation with the grade according to the World Health Organization classification [10]. Salpietro et al. [29], Ildan et al. [30], and Go et al. [25] reported that the degree of edema was positively correlated with tumor invasion into the cortex. Kamitani et al. [31] demonstrated the presence of tumor cells in the thickened arachnoid in the brain parenchyma adjacent to the meningioma after resection.
Meanwhile, tumors with high expression of factors of invasion such as matrix metalloproteinases 2 and 9, exhibit a large amount of peritumoral edema. Therefore, the current theory is based on the expression of vascular permeability and vascular growth factor (VPF/VEGF) [13] in the presence of pial-cerebral substitution or neovascularization.
5. Conclusion
The assumption that all meningiomas behave as benign extra-axial tumors with little effect on the adjacent cerebral cortex seems unsatisfactory. Large meningiomas can cause severe cerebral edema through a variety of mechanisms, but it is not clear which mechanisms play the main role. Many studies have shown the dynamic interaction between meningiomas and neighboring parenchyma (angiogenic factors, matrix metalloproteinases, proliferative activity, pial irrigation patterns and cortical invasion).
Following this line of thought, we believe that both the presence and the duration of peritumoral edema in meningiomas are multifactorial, although tumor size plays an important role.
Limitations
This was a retrospective study with intrinsic bias. In addition, the study did not compare meningiomas that were not operated upon or treated with steroids, which could be useful in defining the importance of the presence of the tumor lesion itself in this particular type of edema. On the other hand, the follow-up time is short since meningiomas usually show slow growth.
Funding
None.
REFERENCES
1. Doga Gurkanlar, Uygur Er, Metin
Sanli, et al. “Peritumoral brain edema in intracranial meningiomas.” J Clin
Neurosci, vol. 12, no. 7, pp. 750-753, 2005. View at: Publisher
Site
| PubMed
2. Sun Ha Paek, Chae-Yong Kim, Young
Yim Kim, et al. Correlation of clinical and biological parameters with
peritumoral edema in meningioma. J Neurooncol, vol. 60, no. 3, pp.
235-245, 2002. View at: Publisher
Site
| PubMed
3. M Bitzer, L Wöckel, M Morgalla, et
al. “Peritumoural brain oedema in intracranial meningiomas: influence of tumour
size, location and histology.” Acta Neurochir (Wien), vol. 139, no. 12,
pp. 1136-1142, 1997. View at: Publisher Site | PubMed
4. Byung-Won Kim, Min-Su Kim, Sang-Woo
Kim, et al. “Peritumoral brain edema in meningiomas: correlation of radiologic
and pathologic features.” J Korean Neurosurg Soc, vol. 49, no. 1, pp. 26-30, 2011. View at: Publisher
Site
| PubMed
5. Jorge E Alvernia, Marc P Sindou
“Preoperative neuroimaging findings as a predictor of the surgical plane of
cleavage: prospective study of 100 consecutive cases of intracranial
meningioma.” J
Neurosurg,
vol. 100, no. 3, pp. 422-430, 2004. View at: Publisher
Site
| PubMed
6. M Ide, M Jimbo, O Kubo, et al.
“Peritumoral brain edema associated with meningioma--histological study of the
tumor margin and surrounding brain.” Neurol Med Chir (Tokyo), vol. 32,
no. 2, pp. 65-71, 1992. View at: Publisher Site | PubMed
7. Tadashi Osawa, Masahiko Tosaka,
Masaya Nagaishi, et al. “Factors affecting peritumoral brain edema in
meningioma: special histological subtypes with prominently extensive edema.” J
Neurooncol, vol. 111, no. 1, pp. 49-57, 2013. View at: Publisher
Site
| PubMed
8. Katharine J Drummond, Jay-Jiguang
Zhu, Peter McL Black “Meningiomas: updating basic science, management, and
outcome.” Neurologist, vol. 10, no. 3, pp. 113-130, 2004. View at: Publisher
Site
| PubMed
9. S N Kalkanis, A Quiñones-Hinojosa, E
Buzney, et al. “Quality
of life following surgery for intracranial meningiomas at Brigham and Women's
Hospital: a study of 164 patients using a modification of the functional
assessment of cancer therapy-brain questionnaire.” J Neurooncol, vol.
48, no. 3, pp. 233-241, 2000. View at: Publisher
Site
| PubMed
10. M Ide, M Jimbo, M Yamamoto, et al.
“MIB-1 staining index and peritumoral brain edema of meningiomas.” Cancer,
vol. 78, no. 1, pp. 133-143, 1996. View at: Publisher Site | PubMed
11. R D Lobato, R Alday, P A Gómez, et al.
“Brain oedema in
patients with intracranial meningioma. Correlation between clinical,
radiological, and histological factors and the presence and intensity of
oedema.” Acta
Neurochir (Wien), vol. 138, no. 5, pp. 485-494, 1996. View at: Publisher Site | PubMed
12. J Philippon, J F Foncin, R Grob, et
al. “Cerebral edema associated with meningiomas: possible role of a
secretory-excretory phenomenon.” Neurosurgery, vol. 14, no. 3, pp. 295-301, 1984. View at: Publisher
Site
| PubMed
13. H Yoshioka, S Hama, E Taniguchi, et
al. “Peritumoral brain edema associated with meningioma: influence of vascular
endothelial growth factor expression and vascular blood supply.” Cancer,
vol. 85, no. 4, pp. 936-944, 1999. View at: Publisher Site | PubMed
14. J M Stevens, J S Ruiz, B E Kendall
“Observations on peritumoral oedema in meningioma. Part II: Mechanisms of oedema
production.” Neuroradiology, vol. 25, no. 3, pp. 125-131, 1983. View at: Publisher Site | PubMed
15. Michihiro Tanaka, Hans G Imhof,
Bernhard Schucknecht, et al. “Correlation between the efferent venous drainage
of the tumor and peritumoral edema in intracranial meningiomas: superselective
angiographic analysis of 25 cases.” J Neurosurg, vol. 104, no. 3, pp.
382-388, 2006. View at: Publisher
Site
| PubMed
16. T Inamura, S Nishio, I Takeshita, et
al. “Peritumoral brain edema in meningiomas--influence of vascular supply on
its development.” Neurosurgery, vol. 31, no. 2, pp. 179-185, 1992. View
at: Publisher Site | PubMed
17. Wael M. Moussa “Predictive value of
brain edema in preoperative computerized tomography scanning on the recurrence
of meningioma.” Alexandria Journal of Medicine, vol. 48, no. 4, pp.
373-379, 2012. View at: Publisher
Site
18. R E Mantle, B Lach, M R Delgado, et
al. “Predicting the probability of meningioma recurrence based on the quanty of
peritumoral brain edema on computerized tomography scanning.” J Neurosurg,
vol. 91, no. 3, pp. 375-383, 1999. View at: Publisher
Site
| PubMed
19. Moncef Berhouma, Thiebaud Picart,
Chloe Dumot, et al. “Alterations of cerebral microcirculation in peritumoral
edema: feasibility of in vivo sidestream dark-field imaging in intracranial
meningiomas.” Neurooncol
Adv,
vol. 2, no. 1, pp. vdaa108, 2020. View at: Publisher
Site
| PubMed
20. C Andersen, J Astrup, C Gyldensted
“Quantitative MR analysis of glucocorticoid effects on peritumoral edema
associated with intracranial meningiomas and metastases.” J Comput Assist
Tomogr, vol. 18, no. 4, pp. 509-518, 1994. View at: Publisher
Site
| PubMed
21. C Andersen, J Astrup, C Gyldensted
“Quantitation of peritumoural oedema and the effect of steroids using
NMR-relaxation time imaging and blood-brain barrier analysis.” Acta
Neurochir Suppl (Wien), vol. 60, pp. 413-415, 1994. View at: Publisher
Site
| PubMed
22. Z Domingo, G Rowe, A M Blamire, et
al. “Role of ischaemia in the genesis of oedema surrounding meningiomas
assessed using magnetic resonance imaging and spectroscopy.” Br J Neurosurg, vol. 12, no. 5, pp. 414-418, 1998. View at: Publisher
Site
| PubMed
23. Andre Simis, Paulo Henrique Pires de
Aguiar, Claudia C Leite, et al. “Peritumoral brain edema in benign meningiomas:
correlation with clinical, radiologic, and surgical factors and possible role
on recurrence.” Surg Neurol, vol. 70, no. 5, pp. 471-477, 2008. View at:
Publisher
Site
| PubMed
24. C K Goldman, S Bharara, C A Palmer, et
al. “Brain edema in
meningiomas is associated with increased vascular endothelial growth factor
expression.” Neurosurgery, vol. 40, no. 6, pp. 1269-1277, 1997. View at: Publisher
Site
| PubMed
25. K G Go, J T Wilmink, W M Molenaar
“Peritumoral brain edema associated with meningiomas.” Neurosurgery, vol. 23, no. 2, pp. 175-179, 1988. View at: Publisher
Site
| PubMed
26. Michio Yamaguchi, Seiya Shirakata,
Syun Yamasaki, et al. “Ischemic Brain Edema and Compression Brain Edema. Water
Content, Blood-Brain Barrier and Circulation.” Stroke, vol. 7, pp.
77-83, 1976. View at: Publisher Site
27. G B Bradac, R Ferszt, A Bender, et
al. “Peritumoral edema in meningiomas. A radiological and histological study.” Neuroradiology,
vol. 28, no. 4, pp. 304-312, 1986. View at: Publisher Site | PubMed
28. F Maiuri, M Gangemi, S Cirillo, et
al. “Cerebral edema associated with meningiomas.” Surg Neurol, vol. 27, no. 1, pp. 64-68, 1987. View at: Publisher
Site
| PubMed
29. F M Salpietro, C Alafaci, S Lucerna,
et al. “Peritumoral edema in meningiomas: microsurgical observations of
different brain tumor interfaces related to computed tomography.” Neurosurgery,
vol. 35, no. 4, pp. 638-642, 1994. View at: Publisher
Site
| PubMed
30. F Ildan, M Tuna, A P Göçer, et al. “Correlation of the relationships of brain-tumor interfaces, magnetic resonance imaging, and angiographic findings to predict cleavage of meningiomas.” J Neurosurg, vol. 91, no. 3, pp. 384-390, 1999. View at: Publisher Site | PubMed
31.H Kamitani, H Masuzawa, I Kanazawa, et al. “Recurrence of convexity meningiomas: tumor cells in the arachnoid membrane.” Surg Neurol, vol.56, no. 4, pp. 228-235, 2001. View at: Publisher Site | PubMed
